2020 Good Quality Palbociclib Ribociclib - Ledipasvir Actone /PVP – CPF
2020 Good Quality Palbociclib Ribociclib - Ledipasvir Actone /PVP – CPF Detail:
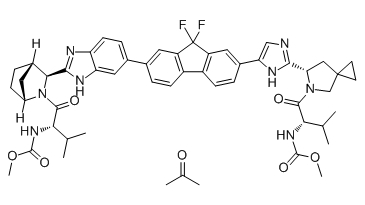
| 雷迪帕韦丙酮/聚维酮 | Ledipasvir actone/PVP | 1256388-51-8 | In-House |
| LPSM1 | 1256387-87-7 | In-House | |
| LPSM1-5 | 291775-59-2 | In-House | |
| LPSM2 | 1129634-44-1 | In-House | |
| LPS-6 | 1441670-89-8 | In-House |
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
Description
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
We have been also concentrating on enhancing the things management and QC method so that we could preserve terrific edge inside the fiercely-competitive enterprise for 2020 Good Quality Palbociclib Ribociclib - Ledipasvir Actone /PVP – CPF , The product will supply to all over the world, such as: Egypt, Mauritius, Iran, We focus on providing service for our clients as a key element in strengthening our long-term relationships. Our continual availability of high grade products in combination with our excellent pre-sale and after-sales service ensures strong competitiveness in an increasingly globalized market.
On this website, product categories is clear and rich, I can find the product I want very quickly and easily, this is really very good!










