2020 High quality Ledipasvir - Thalidomide – CPF
2020 High quality Ledipasvir - Thalidomide – CPF Detail:
Background
Thalidomide was introduced as a sedative drug,immunomodulatory agent and also is investigated for treating symptoms of many cancers.Thalidomide inhibits an E3 ubiquitin ligase, which is a CRBN-DDB1-Cul4A complex.
Description
Thalidomide is initially promoted as a sedative, inhibits cereblon (CRBN), a part of the cullin-4 E3 ubiquitin ligase complex CUL4-RBX1-DDB1, with a Kd of ∼250 nM, and has immunomodulatory, anti-inflammatory and anti-angiogenic cancer properties.
In Vitro
Thalidomide is initially promoted as a sedative, has immunomodulatory, anti-inflammatory and anti-angiogenic cancer properties, and targets cereblon (CRBN), a part of the cullin-4 E3 ubiquitin ligase complex CUL4-RBX1-DDB1, with a Kd of ∼250 nM[1]. Thalidomide (50 μg/mL) potentiates the anti-tumor activity of icotinib against the proliferation of both PC9 and A549 cells, and this effect is correlated with apoptosis and cell migration. In addition, Thalidomide and icotinib inhibits the EGFR and VEGF-R2 pathways in PC9 cells[3].
Thalidomide (100 mg/kg, p.o.) inhibits the collagen deposition, down-regulates the mRNA expression level of α-SMA and collagen I, and significantly reduces the pro-inflammatory cytokines in RILF mice. Thalidomide alleviates RILF via suppression of ROS and down-regulation of TGF-β/Smad pathway dependent on Nrf2 status[2]. Thalidomide (200 mg/kg, p.o.) combined with icotinib shows synergistic anti-tumor effects in nude mice bearing PC9 cells, suppressing tumor growth and promoting tumor death[3].
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
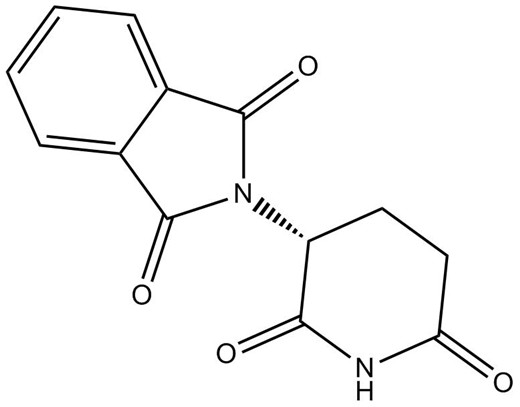
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
Our items are commonly identified and trusted by people and can fulfill repeatedly altering economic and social wants of 2020 High quality Ledipasvir - Thalidomide – CPF , The product will supply to all over the world, such as: Oman, Iraq, Malaysia, We solution have passed through the national skilled certification and been well received in our key industry. Our specialist engineering team will often be ready to serve you for consultation and feedback. We are able to also provide you with no cost samples to meet your needs. Best efforts will be produced to offer you the very best service and solutions. For anyone who is considering our business and solutions, please speak to us by sending us emails or get in touch with us right away. As a way to know our products and enterprise. lot more, you'll be able to come to our factory to find out it. We will constantly welcome guests from around the globe to our firm. o build enterprise. elations with us. Please really feel absolutely free to make contact with us for small business and we believe we will share the top trading practical experience with all our merchants.
A good manufacturers, we have cooperated twice, good quality and good service attitude.











