2020 Latest Design Sofosbuvir 400 Mg Daclatasvir 60 Mg - Baloxavir Marboxil 1985606-14-1 – CPF
2020 Latest Design Sofosbuvir 400 Mg Daclatasvir 60 Mg - Baloxavir Marboxil 1985606-14-1 – CPF Detail:
Background
Baloxavir marboxil is an antiviral drug and is an endonuclease inhibitor. In rats and monkeys, the blood concentration of the drug is lower than the minimum detection amount in a single oral administration of the drug. In preclinical influenza A and B infection models (including strains that are resistant to existing antiviral drugs), Baloxavir marboxil has a certain effect.
Chemical Properties
| Storage | Store at -20°C |
| M.Wt | 571.55 |
| Cas No. | 1985606-14-1 |
| Formula | C27H23F2N3O7S |
| Synonyms | S-033188 |
| Solubility | Soluble in DMSO |
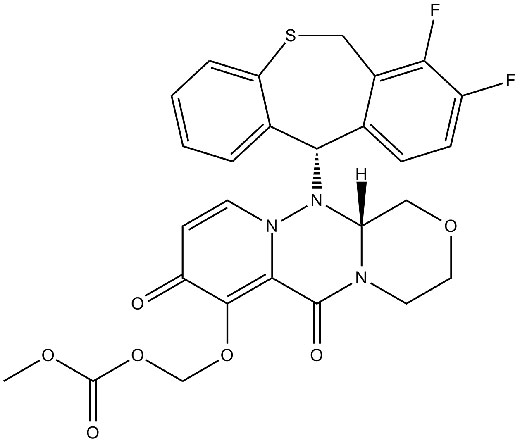
| Chemical Name | (((R)-12-((S)-7,8-difluoro-6,11-dihydrodibenzo[b,e]thiepin-11-yl)-6,8-dioxo-3,4,6,8,12,12a-hexahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin-7-yl)oxy)methyl methyl carbonate |
| SDF | Download SDF |
| Canonical SMILES | O=C1C=CN(N([C@]2(N(C3=O)CCOC2)[H])[C@H]4C5=CC=C(C(F)=C5CSC6=CC=CC=C46)F)C3=C1OCOC(OC)=O |
| Shipping Condition | Evaluation sample solution: ship with blue ice. All other available sizes: ship with RT, or blue ice upon request. |
| General tips | For obtaining a higher solubility, please warm the tube at 37°C and shake it in the ultrasonic bath for a while. Stock solution can be stored below -20°C for several months. |
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
To be a result of ours specialty and repair consciousness, our corporation has won an excellent reputation amongst customers all around the entire world for 2020 Latest Design Sofosbuvir 400 Mg Daclatasvir 60 Mg - Baloxavir Marboxil 1985606-14-1 – CPF , The product will supply to all over the world, such as: The Swiss, Armenia, India, To have much more enterprise. ompanions, we've got updated the item list and seek for optimistic co-operation. Our web-site shows the latest and complete information and facts about our goods list and company. For further acknowledge, our consultant service group in Bulgaria will reply to all of the inquiries and complications immediately. They're going to make their finest effort to meet buyers need. Also we support the delivery of absolutely free samples. Business visits to our business in Bulgaria and factory are generally welcome for a win-win negotiation. Hope to expertise a happy company co-operation perform with you.
Reasonable price, good attitude of consultation, finally we achieve a win-win situation,a happy cooperation!









