2020 wholesale price Daclatasvir Asunaprevir - Ledipasvir Actone /PVP – CPF
2020 wholesale price Daclatasvir Asunaprevir - Ledipasvir Actone /PVP – CPF Detail:
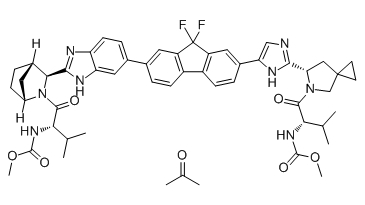
| 雷迪帕韦丙酮/聚维酮 | Ledipasvir actone/PVP | 1256388-51-8 | In-House |
| LPSM1 | 1256387-87-7 | In-House | |
| LPSM1-5 | 291775-59-2 | In-House | |
| LPSM2 | 1129634-44-1 | In-House | |
| LPS-6 | 1441670-89-8 | In-House |
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
Description
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:
Related Product Guide:
With dependable high-quality method, fantastic standing and ideal purchaser assistance, the series of products produced by our firm are exported to many countries and regions for 2020 wholesale price Daclatasvir Asunaprevir - Ledipasvir Actone /PVP – CPF , The product will supply to all over the world, such as: Romania, Mauritius, Greece, All the imported machines effectively control and guarantee the machining precision for the products. Besides, we have a group of high-quality management personnels and professionals, who make the high-quality products and have the ability to develop new products to expand our market home and abroad. We sincerely expect customers come for a blooming business for both of us.
We have been looking for a professional and responsible supplier, and now we find it.










