Bictegravir 1611493-60-7
Description
Bictegravir is a novel, potent inhibitor of HIV-1 integrase with an IC50 of 7.5 nM.
In Vitro
Bictegravir (BIC) inhibits the strand transfer activity with an IC50 of 7.5± 0.3 nM. Relative to its inhibition of strand transfer activity, Bictegravir is a much weaker inhibitor of 3′-processing activity of HIV-1 IN, with an IC50 of 241±51 nM. Bictegravir enhances the accumulation of 2-LTR circles ~5-fold relative to the mock-treated control and reduces the amount of authentic integration products in infected cells by 100-fold. Bictegravir potently inhibits HIV-1 replication in both MT-2 and MT-4 cells with EC50s of 1.5 and 2.4 nM, respectively. Bictegravir exhibits potent antiviral effects in both primary CD4+ T lymphocytes and monocyte-derived macrophages, with EC50s of 1.5±0.3 nM and 6.6±4.1 nM, respectively, which are comparable to values obtained in T-cell lines[1].
MCE has not independently confirmed the accuracy of these methods. They are for reference only.
| NCT Number | Sponsor | Condition | Start Date |
Phase |
| NCT03998176 | University of Nebraska|Gilead Sciences | HIV-1-infection | October 9, 2019 |
Phase 4 |
| NCT03789968 | Thomas Jefferson University|University of Maryland, College Park|Indiana University Health|The Brooklyn Hospital Center|University of Illinois at Chicago|Nova Southeastern University|University of California, San Francisco | HIV+AIDS | September 1, 2019 | |
| NCT04249037 | University of Colorado, Denver|Gilead Sciences | HIV+AIDS | March 1, 2020 |
Not Applicable |
| NCT04132674 | Vancouver Infectious Diseases Centre | Human Immunodeficiency Virus I Infection|Drug Use | November 26, 2018 |
Phase 4 |
| NCT04054089 | Cristina Mussini|University of Modena and Reggio Emilia | HIV Infections | September 2019 |
Phase 4 |
| NCT04155554 | Azienda Ospedaliera Universitaria Senese|Catholic University of the Sacred Heart|Ospedale Policlinico San Martino|Azienda Ospedaliera San Paolo|Ospedale Amedeo di Savoia | HIV-1-infection | January 29, 2020 |
Phase 3 |
| NCT02275065 | Gilead Sciences | HIV-1 Infection | October 2014 |
Phase 1 |
| NCT03711253 | University of Southern California | Acute HIV Infection | October 14, 2019 |
Phase 4 |
| NCT02400307 | Gilead Sciences | HIV | April 17, 2015 |
Phase 1 |
| NCT03499483 | Fenway Community Health | HIV Prevention | January 24, 2019 |
Phase 4 |
| NCT03502005 | Midland Research Group, Inc.|Gilead Sciences | Human Immunodeficiency Virus | March 1, 2018 |
Phase 4 |
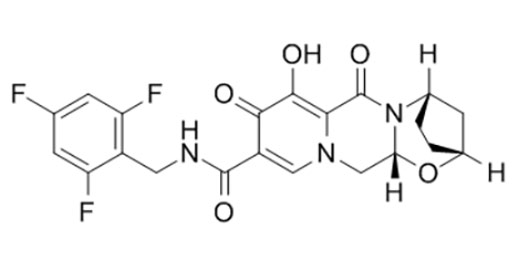
Chemical structure





Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.


Korea Countec Bottled Packaging Line


Taiwan CVC Bottled Packaging Line


Italy CAM Board Packaging Line

German Fette Compacting Machine

Japan Viswill Tablet Detector

DCS Control Room





