China Wholesale Rosuvastatin Calcium En Español Factory - Ledipasvir Actone /PVP – CPF
China Wholesale Rosuvastatin Calcium En Español Factory - Ledipasvir Actone /PVP – CPF Detail:
| 雷迪帕韦丙酮/聚维酮 | Ledipasvir actone/PVP | 1256388-51-8 | In-House |
| LPSM1 | 1256387-87-7 | In-House | |
| LPSM1-5 | 291775-59-2 | In-House | |
| LPSM2 | 1129634-44-1 | In-House | |
| LPS-6 | 1441670-89-8 | In-House |
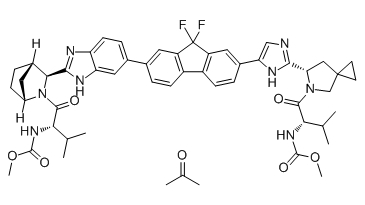
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
Description
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
Every single member from our large efficiency revenue team values customers' wants and company communication for China Wholesale Rosuvastatin Calcium En Español Factory - Ledipasvir Actone /PVP – CPF , The product will supply to all over the world, such as: Houston, Monaco, Pakistan, We have constructed strong and long co-operation relationship with an enormous quantity of companies within this business overseas. Immediate and specialist after-sale service supplied by our consultant group has happy our buyers. Detailed Info and parameters from the merchandise will probably be sent to you for any thorough acknowledge. Free samples may be delivered and company check out to our corporation. n Portugal for negotiation is constantly welcome. Hope to get inquiries type you and construct a long-term co-operation partnership.
This enterprise in the industry is strong and competitive, advancing with the times and develop sustainable, we are very pleased to have a opportunity to cooperate!









