factory customized Tofacitinib Manufacturer - Thalidomide – CPF
factory customized Tofacitinib Manufacturer - Thalidomide – CPF Detail:
Background
Thalidomide was introduced as a sedative drug,immunomodulatory agent and also is investigated for treating symptoms of many cancers.Thalidomide inhibits an E3 ubiquitin ligase, which is a CRBN-DDB1-Cul4A complex.
Description
Thalidomide is initially promoted as a sedative, inhibits cereblon (CRBN), a part of the cullin-4 E3 ubiquitin ligase complex CUL4-RBX1-DDB1, with a Kd of ∼250 nM, and has immunomodulatory, anti-inflammatory and anti-angiogenic cancer properties.
In Vitro
Thalidomide is initially promoted as a sedative, has immunomodulatory, anti-inflammatory and anti-angiogenic cancer properties, and targets cereblon (CRBN), a part of the cullin-4 E3 ubiquitin ligase complex CUL4-RBX1-DDB1, with a Kd of ∼250 nM[1]. Thalidomide (50 μg/mL) potentiates the anti-tumor activity of icotinib against the proliferation of both PC9 and A549 cells, and this effect is correlated with apoptosis and cell migration. In addition, Thalidomide and icotinib inhibits the EGFR and VEGF-R2 pathways in PC9 cells[3].
Thalidomide (100 mg/kg, p.o.) inhibits the collagen deposition, down-regulates the mRNA expression level of α-SMA and collagen I, and significantly reduces the pro-inflammatory cytokines in RILF mice. Thalidomide alleviates RILF via suppression of ROS and down-regulation of TGF-β/Smad pathway dependent on Nrf2 status[2]. Thalidomide (200 mg/kg, p.o.) combined with icotinib shows synergistic anti-tumor effects in nude mice bearing PC9 cells, suppressing tumor growth and promoting tumor death[3].
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
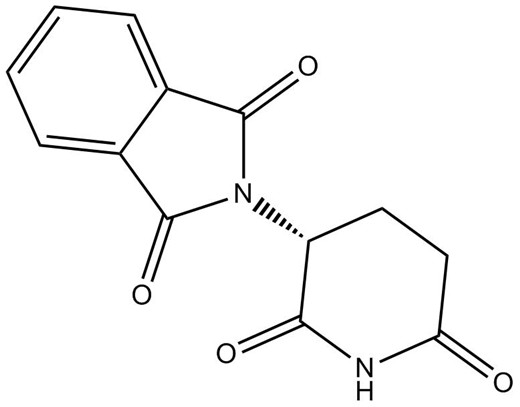
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
To regularly increase the management program by virtue from the rule of "sincerely, good religion and high quality are the base of enterprise development", we greatly absorb the essence of linked products internationally, and constantly produce new goods to satisfy the calls for of shoppers for factory customized Tofacitinib Manufacturer - Thalidomide – CPF , The product will supply to all over the world, such as: Estonia, Bangalore, Dominica, With strong technical strength and advanced production equipment, and SMS people purposefully , professional, dedicated spirit of enterprise. Enterprises took the lead through the ISO 9001:2008 international quality management system certification, CE certification EU ; CCC.SGS.CQC other related product certification. We look forward to reactivating our company connection.
We have been appreciated the Chinese manufacturing, this time also did not let us disappoint,good job!











