Fixed Competitive Price Ticagrelor Classification - LCZ696(Sacubitril + Valsartan) – CPF
Fixed Competitive Price Ticagrelor Classification - LCZ696(Sacubitril + Valsartan) – CPF Detail:
Description
LCZ696 (Sacubitril/Valsartan), comprised Valsartan (an ARB) and Sacubitril (AHU377) in 1:1 molar ratio, is a first-in-class, orally bioavailable, and dual-acting angiotensin receptor-neprilysin (ARN) inhibitor for hypertension and heart failure[1][2][3]. LCZ696 ameliorates diabetic cardiomyopathy by inhibiting inflammation, oxidative stress and apoptosis.
Background
LCZ696 is a first in class ARNi (angiotensin receptor neprilysin inhibitor) comprising anionic moieties of AR valsartan and the neprilysin inhibitor prodrug AHU377 (1:1 ratio) for heart failure and hypertension.
The angiotensin receptors are G-protein-coupled receptors. They mediate the cardiovascular and other effects of angiotensin II which is a bioactive peptide of the renin–angiotensin system. Neprilysin is a neutral endopeptidase that degrades endogenous vasoactive peptides such as natriuretic peptides. Inhibition of neprilysin increases the natriuretic peptides concentration that contributed to cardiac, vascular and renal protection. [1]
In Sprague-Dawley rats, oral administration of LCZ696 led to a dose-dependent rise in immunoreactivity of atrial natriuretic peptide resulting from neprilysin inhibition. In hypertensive double transgenic rats, LCZ696 caused a dose-dependent and sustained reduction in mean arterial pressure. A healthy participants, a randomized, double-blind, placebo-controlled study confirmed that LCZ696 provided concurrent neprilysin inhibition and AT1 receptor blockade. LCZ696 was safe and well tolerated in human. [2] [3]
References:
McMurray JJ, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014 Sep 11;371(11):993-1004.
Gu J, Noe A, Chandra P, Al-Fayoumi S et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010 Apr;50(4):401-14.
Langenickel TH, Dole WP. Angiotensin receptor-neprilysin inhibition with LCZ696: a novel approach for the treatment of heart failure, Drug Discov Today: Ther Strategies (2014),
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
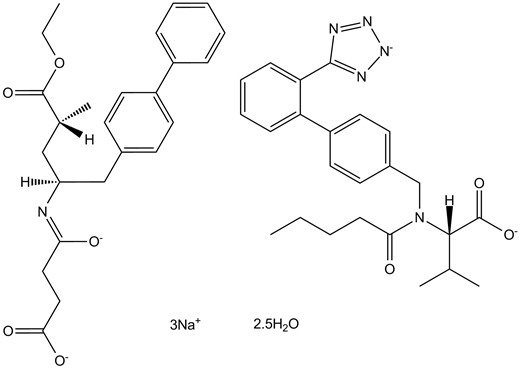
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
We also offer product sourcing and flight consolidation services. We have our own factory and sourcing office. We can provide you with almost every type of product related to our product range for Fixed Competitive Price Ticagrelor Classification - LCZ696(Sacubitril + Valsartan) – CPF , The product will supply to all over the world, such as: Indonesia, Macedonia, New York, With the spirit of "credit first, development through innovation, sincere cooperation and joint growth", our company is striving to create a brilliant future with you, so as to become a most valuable platform for exporting our products in China!
After the signing of the contract, we received satisfactory goods in a short term, this is a commendable manufacturer.










