Free sample for Doxycycline Monohydrate For Chlamydia - Apixaban – CPF
Free sample for Doxycycline Monohydrate For Chlamydia - Apixaban – CPF Detail:
Background
Apixaban is a highly selective and reversible inhibitor of Factor Xa with Ki values of 0.08 nM and 0.17 nM in human and rabbit, respectively[1].
Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. Factor X is activated, by hydrolysis, into factor Xa by both factor IX. Factor Xa is the activated form of the coagulation factorthrombokinase.Inhibiting Factor Xa could offer an alternate method for anticoagulation. Direct Xa inhibitors are popular anticoagulants [2].
In vitro: Apixabanhas exhibited a high degree of potency, selectivity, and efficacy on Factor Xa with Ki of 0.08 nM and 0.17 nM for Human Factor Xa and Rabbit Factor Xa, respectively [1]. Apixaban prolonged the clotting times of normal human plasma with the concentrations (EC2x) of 3.6, 0.37, 7.4 and 0.4 μM, which are required respectively to double the prothrombin time (PT), modified prothrombin time (mPT), activated partial thromboplastin time (APTT) and HepTest. Besides, Apixaban showed the highest potency in human and rabbit plasma, but less potency in rat and dog plasma in both the PT and APTT assays [3].
In vivo: Apixaban exihibited the excellent pharmacokinetics with very low clearance (Cl: 0.02 L kg-1h-1), and low volume of distribution (Vdss: 0.2 L/kg) in the dog. Besides, Apixaban also showed a moderate half-life with T1/2 of 5.8 hours and good oral bioavailability (F: 58%) [1]. In the arteriovenous-shunt thrombosis (AVST), venous thrombosis (VT) and electrically mediated carotid arterial thrombosis (ECAT) rabbit models, Apixaban produced antithrombotic effects with EC50 of 270 nM, 110 nM and 70 nM in a dose-dependent manner[3]. Apixaban significantly inhibited factor Xa activity with an IC50 of 0.22 μM in rabbit ex vivo[4]. In chimpanzee, Apixaban also showed small volume of distribution (Vdss: 0.17 L kg-1), low systemic clearance (Cl: 0.018 L kg-1h-1), and good oral bioavailability (F: 59%) [5].
References:
Pinto D J P, Orwat M J, Koch S, et al. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl) phenyl)-4, 5, 6, 7-tetrahydro-1 H-pyrazolo [3, 4-c] pyridine-3-carboxamide (Apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa[J]. Journal of medicinal chemistry, 2007, 50(22): 5339-5356.
Sidhu P S. Direct Factor Xa Inhibitors as Anticoagulants[J].
Wong P C, Crain E J, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostaticstudies[J]. Journal of Thrombosis and Haemostasis, 2008, 6(5): 820-829.
Zhang D, He K, Raghavan N, et al. Metabolism, pharmacokinetics and pharmacodynamics of the factor Xa inhibitor apixaban in rabbits[J]. Journal of thrombosis and thrombolysis, 2010, 29(1): 70-80.
He K, Luettgen J M, Zhang D, et al. Preclinical pharmacokinetics and pharmacodynamics of apixaban, a potent and selective factor Xa inhibitor[J]. European journal of drug metabolism and pharmacokinetics, 2011, 36(3): 129-139.
Apixaban is a highly selective and reversible inhibitor of Factor Xa with Ki values of 0.08 nM and 0.17 nM in human and rabbit, respectively[1].
Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. Factor X is activated, by hydrolysis, into factor Xa by both factor IX. Factor Xa is the activated form of the coagulation factorthrombokinase.Inhibiting Factor Xa could offer an alternate method for anticoagulation. Direct Xa inhibitors are popular anticoagulants [2].
In vitro: Apixabanhas exhibited a high degree of potency, selectivity, and efficacy on Factor Xa with Ki of 0.08 nM and 0.17 nM for Human Factor Xa and Rabbit Factor Xa, respectively [1]. Apixaban prolonged the clotting times of normal human plasma with the concentrations (EC2x) of 3.6, 0.37, 7.4 and 0.4 μM, which are required respectively to double the prothrombin time (PT), modified prothrombin time (mPT), activated partial thromboplastin time (APTT) and HepTest. Besides, Apixaban showed the highest potency in human and rabbit plasma, but less potency in rat and dog plasma in both the PT and APTT assays [3].
In vivo: Apixaban exihibited the excellent pharmacokinetics with very low clearance (Cl: 0.02 L kg-1h-1), and low volume of distribution (Vdss: 0.2 L/kg) in the dog. Besides, Apixaban also showed a moderate half-life with T1/2 of 5.8 hours and good oral bioavailability (F: 58%) [1]. In the arteriovenous-shunt thrombosis (AVST), venous thrombosis (VT) and electrically mediated carotid arterial thrombosis (ECAT) rabbit models, Apixaban produced antithrombotic effects with EC50 of 270 nM, 110 nM and 70 nM in a dose-dependent manner[3]. Apixaban significantly inhibited factor Xa activity with an IC50 of 0.22 μM in rabbit ex vivo[4]. In chimpanzee, Apixaban also showed small volume of distribution (Vdss: 0.17 L kg-1), low systemic clearance (Cl: 0.018 L kg-1h-1), and good oral bioavailability (F: 59%) [5].
References:
Pinto D J P, Orwat M J, Koch S, et al. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl) phenyl)-4, 5, 6, 7-tetrahydro-1 H-pyrazolo [3, 4-c] pyridine-3-carboxamide (Apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa[J]. Journal of medicinal chemistry, 2007, 50(22): 5339-5356.
Sidhu P S. Direct Factor Xa Inhibitors as Anticoagulants[J].
Wong P C, Crain E J, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostaticstudies[J]. Journal of Thrombosis and Haemostasis, 2008, 6(5): 820-829.
Zhang D, He K, Raghavan N, et al. Metabolism, pharmacokinetics and pharmacodynamics of the factor Xa inhibitor apixaban in rabbits[J]. Journal of thrombosis and thrombolysis, 2010, 29(1): 70-80.
He K, Luettgen J M, Zhang D, et al. Preclinical pharmacokinetics and pharmacodynamics of apixaban, a potent and selective factor Xa inhibitor[J]. European journal of drug metabolism and pharmacokinetics, 2011, 36(3): 129-139.
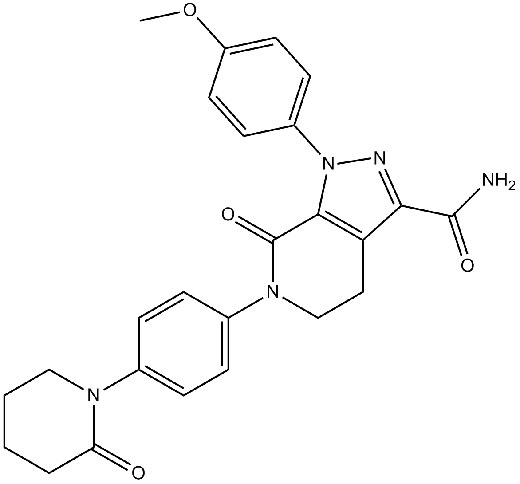
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
Our products and solutions are greatly recognized and trustworthy by customers and may fulfill constantly changing financial and social requires for Free sample for Doxycycline Monohydrate For Chlamydia - Apixaban – CPF , The product will supply to all over the world, such as: Marseille, Cape Town, Lithuania, "Good quality, Good service " is always our tenet and credo. We take every effort to control the quality, package, labels etc and our QC will check every detail during producing and before shipment. We are willing to establish long business relationship with those who seek the high quality products and good service. We have set up a wide sales network across European countries, North of America, South of America, Middle East, Africa, East Asia countries.Please contact us now, you will find our professional experience and high quality grades will contribute to your business.
The supplier cooperation attitude is very good, encountered various problems, always willing to cooperate with us, to us as the real God.










