Good User Reputation for Thalidomide Malformation - Thalidomide – CPF
Good User Reputation for Thalidomide Malformation - Thalidomide – CPF Detail:
Background
Thalidomide was introduced as a sedative drug,immunomodulatory agent and also is investigated for treating symptoms of many cancers.Thalidomide inhibits an E3 ubiquitin ligase, which is a CRBN-DDB1-Cul4A complex.
Description
Thalidomide is initially promoted as a sedative, inhibits cereblon (CRBN), a part of the cullin-4 E3 ubiquitin ligase complex CUL4-RBX1-DDB1, with a Kd of ∼250 nM, and has immunomodulatory, anti-inflammatory and anti-angiogenic cancer properties.
In Vitro
Thalidomide is initially promoted as a sedative, has immunomodulatory, anti-inflammatory and anti-angiogenic cancer properties, and targets cereblon (CRBN), a part of the cullin-4 E3 ubiquitin ligase complex CUL4-RBX1-DDB1, with a Kd of ∼250 nM[1]. Thalidomide (50 μg/mL) potentiates the anti-tumor activity of icotinib against the proliferation of both PC9 and A549 cells, and this effect is correlated with apoptosis and cell migration. In addition, Thalidomide and icotinib inhibits the EGFR and VEGF-R2 pathways in PC9 cells[3].
Thalidomide (100 mg/kg, p.o.) inhibits the collagen deposition, down-regulates the mRNA expression level of α-SMA and collagen I, and significantly reduces the pro-inflammatory cytokines in RILF mice. Thalidomide alleviates RILF via suppression of ROS and down-regulation of TGF-β/Smad pathway dependent on Nrf2 status[2]. Thalidomide (200 mg/kg, p.o.) combined with icotinib shows synergistic anti-tumor effects in nude mice bearing PC9 cells, suppressing tumor growth and promoting tumor death[3].
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
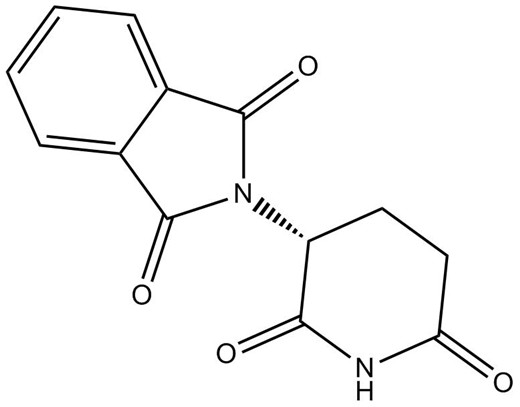
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
To be a result of ours specialty and service consciousness, our enterprise has won an excellent status between buyers all around the globe for Good User Reputation for Thalidomide Malformation - Thalidomide – CPF , The product will supply to all over the world, such as: Sydney, Rio de Janeiro, Boston, We supply skilled service, prompt reply, timely delivery, excellent quality and best price to our customers. Satisfaction and good credit to every customer is our priority. We focus on every detail of order processing for customers till they have received safe and sound items with good logistics service and economical cost. Depending on this, our products and solutions are sold very well in the countries in Africa, the Mid-East and Southeast Asia. Adhering to the business philosophy of ??customer first, forge ahead', we sincerely welcome clients from at home and abroad to cooperate with us.
We have been engaged in this industry for many years, we appreciate the work attitude and production capacity of the company, this is a reputable and professional manufacturer.









