Hot Selling for Sofosbuvir And Daclatasvir - Ledipasvir Actone /PVP – CPF
Hot Selling for Sofosbuvir And Daclatasvir - Ledipasvir Actone /PVP – CPF Detail:
| 雷迪帕韦丙酮/聚维酮 | Ledipasvir actone/PVP | 1256388-51-8 | In-House |
| LPSM1 | 1256387-87-7 | In-House | |
| LPSM1-5 | 291775-59-2 | In-House | |
| LPSM2 | 1129634-44-1 | In-House | |
| LPS-6 | 1441670-89-8 | In-House |
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
Description
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
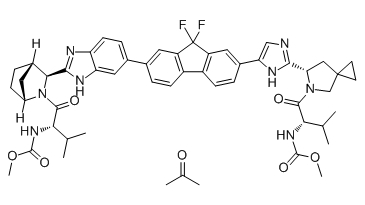
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
We are experienced manufacturer. Wining the majority of the crucial certifications of its market for Hot Selling for Sofosbuvir And Daclatasvir - Ledipasvir Actone /PVP – CPF , The product will supply to all over the world, such as: Yemen, Honduras, Cairo, We have top engineers in these industries and an efficient team in the research. What is more, we have our own archives mouths and markets in China at low cost. Therefore, we can meet different inquiries from different clients. Please find our website to check more information from our products.
The customer service staff is very patient and has a positive and progressive attitude to our interest, so that we can have a comprehensive understanding of the product and finally we reached an agreement, thanks!










