Newly Arrival Thalidomide Adverse Effects - Niraparib 1038915-60-4 – CPF
Newly Arrival Thalidomide Adverse Effects - Niraparib 1038915-60-4 – CPF Detail:
Description
Niraparib (MK-4827) is a highly potent and orally bioavailable PARP1 and PARP2 inhibitor with IC50s of 3.8 and 2.1 nM, respectively. Niraparib leads to inhibition of repair of DNA damage, activates apoptosis and shows anti-tumor activity.
In Vitro
Niraparib (MK-4827) inhibits PARP activity with EC50=4 nM and EC90=45 nM in a whole cell assay. MK-4827 inhibits proliferation of cancer cells with mutant BRCA-1 and BRCA-2 with CC50 in the 10-100 nM range. MK-4827 displays excellent PARP 1 and 2 inhibition with IC50=3.8 and 2.1 nM, respectively, and in a whole cell assay[1]. To validate that Niraparib (MK-4827) inhibits PARP in these cell lines, A549 and H1299 cells are treated with 1 μM MK-4827 for various times and measured PARP enzymatic activity using a chemiluminescent assay. The results show that Niraparib (MK-4827) inhibits PARP within 15 minutes of treatment reaching about 85% inhibition in the A549 cells at 1 h and about 55% inhibition at 1 h for the H1299 cells.
Niraparib (MK-4827) is well tolerated and demonstrates efficacy as a single agent in a xenograft model of BRCA-1 deficient cancer. Niraparib (MK-4827) is well tolerated in vivo and demonstrates efficacy as a single agent in a xenograft model of BRCA-1 deficient cancer. Niraparib (MK-4827) is characterized by acceptable pharmacokinetics in rats with plasma clearance of 28 (mL/min)/kg, very high volume of distribution (Vdss=6.9 L/kg), long terminal half-life (t1/2=3.4 h), and excellent bioavailability, F=65%[1]. Niraparib (MK-4827) enhances radiation response of p53 mutant Calu-6 tumor in both cases, with the single daily dose of 50 mg/kg being more effective than 25 mg/kg given twice daily].
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
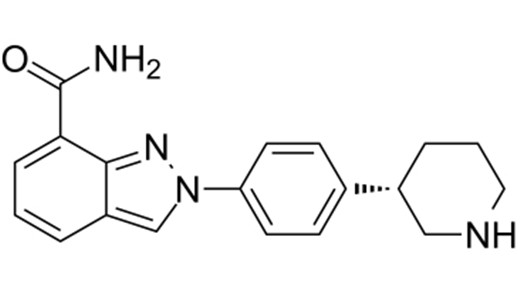
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
We strive for excellence, service the customers", hopes to become the best cooperation team and dominator enterprise for personnel, suppliers and customers, realizes value share and continuous promotion for Newly Arrival Thalidomide Adverse Effects - Niraparib 1038915-60-4 – CPF , The product will supply to all over the world, such as: Brisbane, Turin, Jordan, We take measure at any expense to achieve essentially the most up-to-date equipment and approaches. The packing of nominated brand is our a further distinguishing feature. The products to assure years of trouble-free service has attracted a great deal customers. The solutions are obtainable in improved designs and richer assortment, they're created scientifically of purely raw supplies. It readily available in a variety of designs and specifications for your selection. The most recent kinds are a great deal better than the preceding one particular and they are quite popular with lots of prospects.
The sales person is professional and responsible, warm and polite, we had a pleasant conversation and no language barriers on communication.










