OEM Manufacturer Daclatasvir Tablets 60 Mg - Baloxavir Marboxil 1985606-14-1 – CPF
OEM Manufacturer Daclatasvir Tablets 60 Mg - Baloxavir Marboxil 1985606-14-1 – CPF Detail:
Background
Baloxavir marboxil is an antiviral drug and is an endonuclease inhibitor. In rats and monkeys, the blood concentration of the drug is lower than the minimum detection amount in a single oral administration of the drug. In preclinical influenza A and B infection models (including strains that are resistant to existing antiviral drugs), Baloxavir marboxil has a certain effect.
Chemical Properties
| Storage | Store at -20°C |
| M.Wt | 571.55 |
| Cas No. | 1985606-14-1 |
| Formula | C27H23F2N3O7S |
| Synonyms | S-033188 |
| Solubility | Soluble in DMSO |
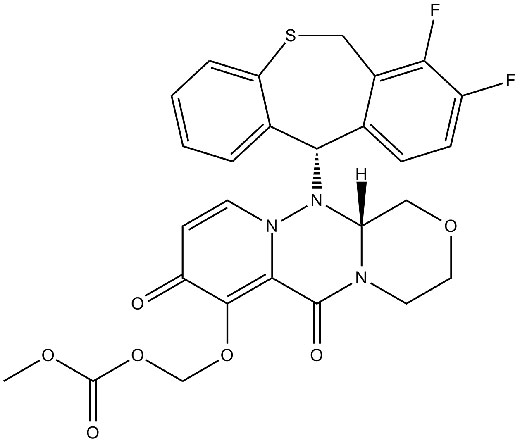
| Chemical Name | (((R)-12-((S)-7,8-difluoro-6,11-dihydrodibenzo[b,e]thiepin-11-yl)-6,8-dioxo-3,4,6,8,12,12a-hexahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin-7-yl)oxy)methyl methyl carbonate |
| SDF | Download SDF |
| Canonical SMILES | O=C1C=CN(N([C@]2(N(C3=O)CCOC2)[H])[C@H]4C5=CC=C(C(F)=C5CSC6=CC=CC=C46)F)C3=C1OCOC(OC)=O |
| Shipping Condition | Evaluation sample solution: ship with blue ice. All other available sizes: ship with RT, or blue ice upon request. |
| General tips | For obtaining a higher solubility, please warm the tube at 37°C and shake it in the ultrasonic bath for a while. Stock solution can be stored below -20°C for several months. |
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
Our company sticks into the basic principle of "Quality is definitely the life of the business, and status may be the soul of it" for OEM Manufacturer Daclatasvir Tablets 60 Mg - Baloxavir Marboxil 1985606-14-1 – CPF , The product will supply to all over the world, such as: Nairobi, Bulgaria, Buenos Aires, We have a professional sales team, they have mastered the best technology and manufacturing processes, have years of experience in foreign trade sales, with customers able to communicate seamlessly and accurately understand the real needs of customers, providing customers with personalized service and unique products.
The factory workers have a good team spirit, so we received high quality products fast, in addition, the price is also appropriate, this is a very good and reliable Chinese manufacturers.









