OEM manufacturer Pregabalin Manufacturer - Sofosbuvir – CPF
OEM manufacturer Pregabalin Manufacturer - Sofosbuvir – CPF Detail:
| 索非布韦 | Sofosbuvir | 1190307-88-0 | In-House |
| SFBM | 1256490-31-9 | In-House | |
| SFB-8 | 874638-80-9 | In-House | |
| SFB-10 | 817204-32-3 | In-House | |
| SFBA-1 | 863329-66-2 | In-House | |
| SFBMA | 1334513-02-8 | In-House |
Generic Name: sofosbuvir (soe FOS bue vir)
Brand Names: Sovaldi
Sofosbuvir is an antiviral medicine that is used to treat chronic hepatitis C in adults and children who are at least 3 years old and an HCV RNA replication inhibitor with an EC50 of 92 nM.
Sofosbuvir must be given in combination with other antiviral medications (usually ribavirin with or without peginterferon alfa). Sofosbuvir should not be used alone.
Sofosbuvir treats specific genotypes of hepatitis C, and only in certain people. Use only the medications prescribed for you. Do not share your medicine with other people.
Sofosbuvir is sometimes used in people who also have HIV, or people who have liver cancer and are going to have a liver transplant. This medicine is not a treatment for HIV or AIDS.
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
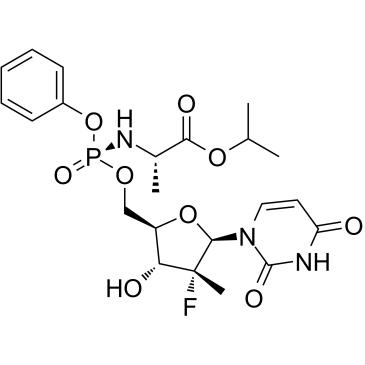
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
We have been proud from the higher consumer gratification and wide acceptance due to our persistent pursuit of high quality both on product or service and service for OEM manufacturer Pregabalin Manufacturer - Sofosbuvir – CPF , The product will supply to all over the world, such as: Puerto Rico, Cologne, Auckland, Being the top solutions of our factory, our solutions series have been tested and won us experienced authority certifications. For additional parameters and item list details, be sure to click the button to acquire additional nformation.
The company account manager has a wealth of industry knowledge and experience, he could provide appropriate program according our needs and speak English fluently.










