OEM/ODM Factory Ledipasvir Actone - Sofosbuvir – CPF
OEM/ODM Factory Ledipasvir Actone - Sofosbuvir – CPF Detail:
| 索非布韦 | Sofosbuvir | 1190307-88-0 | In-House |
| SFBM | 1256490-31-9 | In-House | |
| SFB-8 | 874638-80-9 | In-House | |
| SFB-10 | 817204-32-3 | In-House | |
| SFBA-1 | 863329-66-2 | In-House | |
| SFBMA | 1334513-02-8 | In-House |
Generic Name: sofosbuvir (soe FOS bue vir)
Brand Names: Sovaldi
Sofosbuvir is an antiviral medicine that is used to treat chronic hepatitis C in adults and children who are at least 3 years old and an HCV RNA replication inhibitor with an EC50 of 92 nM.
Sofosbuvir must be given in combination with other antiviral medications (usually ribavirin with or without peginterferon alfa). Sofosbuvir should not be used alone.
Sofosbuvir treats specific genotypes of hepatitis C, and only in certain people. Use only the medications prescribed for you. Do not share your medicine with other people.
Sofosbuvir is sometimes used in people who also have HIV, or people who have liver cancer and are going to have a liver transplant. This medicine is not a treatment for HIV or AIDS.
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
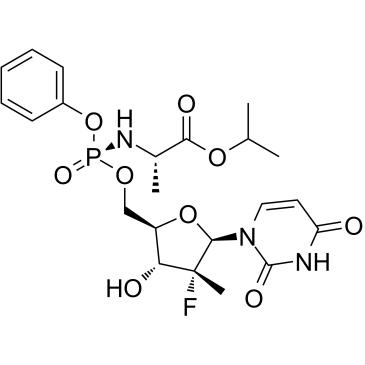
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
With a sound enterprise credit history, exceptional after-sales services and modern production facilities, we've earned an outstanding track record amongst our consumers across the whole world for OEM/ODM Factory Ledipasvir Actone - Sofosbuvir – CPF , The product will supply to all over the world, such as: Milan, Albania, Albania, We've been always creating new technology to streamline the production, and give products with competitive prices and high quality! Customer satisfaction is our priority! You can let us know your idea to develop unique design for your own model to prevent too much similar parts in the market! We are going to present our best service to satisfy all your needs! Remember to contact us right away!
This manufacturers not only respected our choice and requirements, but also gave us a lot of good suggestions, ultimately, we successfully completed the procurement tasks.









