OEM/ODM Factory Olmesartan Hydrochlorothiazide Generic - Atorvastatin Calcium – CPF
OEM/ODM Factory Olmesartan Hydrochlorothiazide Generic - Atorvastatin Calcium – CPF Detail:
Background
Atorvastatin Calcium is a potent inhibitor of HMG-CoA reductase with IC50 value of 150 nM[1].
HMG-CoA reductase is the key enzyme of the mevalonate pathway which produces cholesterol. HMG-CoA is the rate-limiting enzyme and is important for lowering the blood cholesterol levels. HMG-CoA reductase is located in the endoplasmic reticulum and contains eight transmembrane domains. The inhibitors of HMG-CoA reductase can induce the LDL (low density lipoprotein) receptors expression in the liver. It leads to increase the catabolism levels of plasma LDL and lower the concentration of plasma cholesterol which is an important determinant of atherosclerosis. HMG-CoA reductase plays an important role in cholesterol synthesis. HMG-CoA is the ony target for cholesterol-lowering drugs. HMG-CoA reductase is also an important enzyme for development. The activity of HMG-CoA reductase is related to germ cell migration defects. Inhibition of its activity can leadto intracerebral hemorrhage[1].
Atorvastatin is an HMG-CoA reductase inhibitor with IC50 value of 154 nM. It is effective in treating certain dyslipidemias and hypercholesterolemia[1]. Atorvastatin treatment at 40 mg decreases total cholesterol of 40% after 40 days.[1] It also be used to treat coronary or stroke patients with normal cholesterol levels.[2] Atorvastatin also decreases low density lipoprotein apheresis in patients by inducing LDL-receptors expression.
It is metabolized to several metabolites which are important for the effect of the therapeutic actions by CYP3A4 (cytochrome P450 3A4).[3]
References:
[1]. van Dam M, Zwart M, de Beer F, Smelt AH, Prins MH, Trip MD, Havekes LM, Lansberg PJ, Kastelein JJ: Long term efficacy and safety of atorvastatin in the treatment of severe type III and combined dyslipidaemia. Heart 2002, 88(3):234-238.
[2]. Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT et al: Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003, 361(9364):1149-1158.
[3]. Lennernas H: Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet 2003, 42(13):1141-1160.
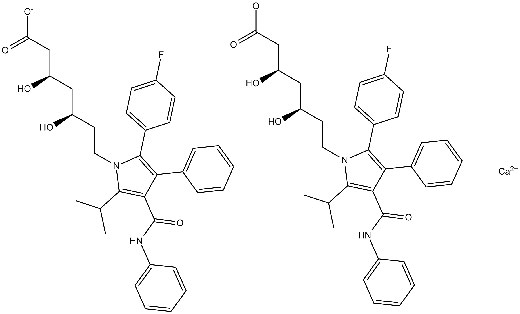
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
Assume full duty to satisfy all demands of our clients; reach steady advancements by marketing the development of our purchasers; grow to be the final permanent cooperative partner of clientele and maximize the interests of customers for OEM/ODM Factory Olmesartan Hydrochlorothiazide Generic - Atorvastatin Calcium – CPF , The product will supply to all over the world, such as: Madrid, United Arab Emirates, Norwegian, With the highest standards of product quality and service, our products have been exported to more than 25 countries like the USA, CANADA, GERMANY, FRANCE, UAE, Malaysia and so on.We are very pleased to serve customers from all over the world!
This supplier's raw material quality is stable and reliable, has always been in accordance with the requirements of our company to provide the goods that quality meet our requirements.











