OEM/ODM Supplier Hydrochlorothiazide Tablet - Ledipasvir Actone /PVP – CPF
OEM/ODM Supplier Hydrochlorothiazide Tablet - Ledipasvir Actone /PVP – CPF Detail:
| 雷迪帕韦丙酮/聚维酮 | Ledipasvir actone/PVP | 1256388-51-8 | In-House |
| LPSM1 | 1256387-87-7 | In-House | |
| LPSM1-5 | 291775-59-2 | In-House | |
| LPSM2 | 1129634-44-1 | In-House | |
| LPS-6 | 1441670-89-8 | In-House |
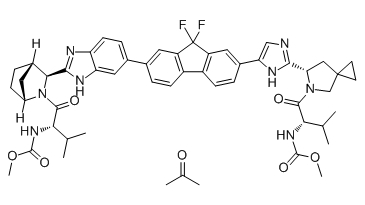
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
Description
Ledipasvir acetone (GS-5885 acetone) is the active ingredient of Ledipasvir. Ledipasvir is an inhibitor of the hepatitis C virus NS5A, with EC50 values of 34 pM against GT1a and 4 pM against GT1b replicon.
In Vitro
Ledipasvir acetone is considered the active ingredient, which is converted to Ledipasvir spray-dried dispersion, an amorphous free base.
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
Our pursuit and firm aim should be to "Always fulfill our buyer requirements". We carry on to produce and structure top-quality excellent solutions for equally our aged and new consumers and accomplish a win-win prospect for our consumers as well as us for OEM/ODM Supplier Hydrochlorothiazide Tablet - Ledipasvir Actone /PVP – CPF , The product will supply to all over the world, such as: Buenos Aires, The Swiss, Philadelphia, Each year, many of our customers would visit our company and achieve great business advancements working with us. We sincerely welcome you to visit us at any time and together we will prevail to a greater success in the hair industry.
We have worked with many companies, but this time is the best,detailed explanation, timely delivery and quality qualified, nice!








