OEM/ODM Supplier Pitavastatin Manufacturer - Ribociclib 1374639-75-4 – CPF
OEM/ODM Supplier Pitavastatin Manufacturer - Ribociclib 1374639-75-4 – CPF Detail:
Description
Ribociclib (LEE01) is a highly specific CDK4/6 inhibitor with IC50 values of 10 nM and 39 nM, respectively, and is over 1,000-fold less potent against the cyclin B/CDK1 complex.
In Vitro
Treating a panel of 17 neuroblastoma cell lines with Ribociclib (LEE011) across a four-log dose range (10 to 10,000 nM). Treatment with Ribociclib significantly inhibits substrate adherent growth relative to the control in 12 of the 17 neuroblastoma cell lines examined (mean IC50=306±68 nM, considering sensitive lines only, where sensitivity is defined as an IC50 of less than 1 μM. Ribociclib treatment of two neuroblastoma cell lines (BE2C and IMR5) with demonstrated sensitivity to CDK4/6 inhibition results in a dose-dependent accumulation of cells in the G0/G1 phase of the cell cycle. This G0/G1 arrest becomes significant at Ribociclib concentrations of 100 nM (p=0.007) and 250 nM (p=0.01), respectively.
CB17 immunodeficient mice bearing BE2C, NB-1643 (MYCN amplified, sensitive in vitro), or EBC1 (non-amplified, resistant in vitro) xenografts are treated once daily for 21 days with Ribociclib (LEE011; 200 mg/kg) or with a vehicle control. This dosing strategy is well tolerated, as no weight loss or other signs of toxicity are observed in any of the xenograft models. Tumor growth is significantly delayed throughout the 21 days of treatment in mice harboring the BE2C or 1643 xenografts (both, p<0.0001), although growth resumed post-treatment.
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
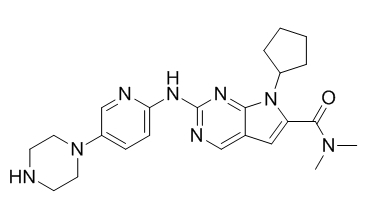
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
We offer fantastic strength in high quality and enhancement,merchandising,income and marketing and procedure for OEM/ODM Supplier Pitavastatin Manufacturer - Ribociclib 1374639-75-4 – CPF , The product will supply to all over the world, such as: Portland, Canberra, Oman, If any item be of interest to you, you should let us know. We will try our best to satisfy your requirements with high quality goods, the best prices and prompt delivery. You should feel free to contact us at any time. We will reply you when we receive your inquiries. Make sure you note that samples are available before we start our business.
Although we are a small company, we are also respected. Reliable quality, sincere service and good credit, we are honored to be able to work with you!










