Quality Inspection for Sofosbuvir Daclatasvir Dose - Folic Acid – CPF
Quality Inspection for Sofosbuvir Daclatasvir Dose - Folic Acid – CPF Detail:
Background
Extracted from Spinacia oleracea;Store the product in sealed, cool and dry condition
Description
Folic acid(Vitamin M; Vitamin B9) is a B vitamin; is necessary for the production and maintenance of new cells, for DNA synthesis and RNA synthesis.
Clinical Trial
| NCT Number | Sponsor | Condition | Start Date |
Phase |
| NCT03332602 | Swiss Federal Institute of Technology | Iron-deficiency | April 4, 2018 |
Not Applicable |
Storage
4°C, protect from light
*In solvent : -80°C, 6 months; -20°C, 1 month (protect from light)
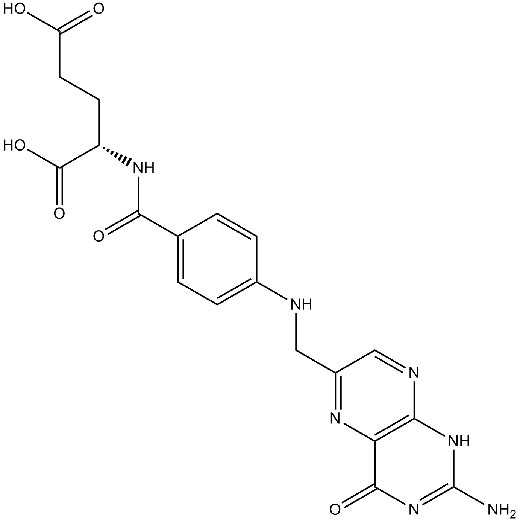
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:
Related Product Guide:
We have a highly efficient team to deal with inquiries from customers. Our goal is "100% customer satisfaction by our product quality, price & our team service" and enjoy a good reputation among clients. With many factories, we can provide a wide range of Quality Inspection for Sofosbuvir Daclatasvir Dose - Folic Acid – CPF , The product will supply to all over the world, such as: USA, kazan, Rome, We now have been making our goods for more than 20 years . Mainly do wholesale , so we've the most competitive price , but highest quality. For the past years , we got very good feedbacks , not only because we offer good solutions , but also because of our good after-sale service . We are here waiting for yourself for your inquiry.
The goods we received and the sample sales staff display to us have the same quality, it is really a creditable manufacturer.