Reasonable price for Velpatasvir Manufacturer - Relugolix 737789-87-6 – CPF
Reasonable price for Velpatasvir Manufacturer - Relugolix 737789-87-6 – CPF Detail:
Relugolix is used to treat prostate cancer in men.
Brand Names: Orgovyx
Drug Class: Antineoplastic - LHRH (GnRH) Antagonist Pituitary Suppressants
Availability: Prescription Required
Pregnancy: Consult your doctor. This medication may be harmful to an unborn child.
Lactation: Consult a doctor before using
Relugolix is an orally available, non-peptide gonadotropin-releasing hormone (GnRH or luteinizing hormone-releasing hormone (LHRH)) antagonist, with potential antineoplastic activity. Relugolix competitively binds to and blocks the GnRH receptor in the anterior pituitary gland, which both prevents GnRH binding to the GnRH receptor and inhibits the secretion and release of both luteinizing hormone (LH) and follicle stimulating hormone (FSH). In males, the inhibition of LH secretion prevents the release of testosterone from Leydig cells in the testes. Since testosterone is required to sustain prostate growth, reducing testosterone levels may inhibit hormone-dependent prostate cancer cell proliferation.
Relugolix is a gonadotropin-releasing hormone (GnRH) receptor antagonist used in the treatment of several hormone-responsive conditions. It was first approved in Japan in 2019, under the brand name Relumina, for the symptomatic treatment of uterine fibroids, and more recently by the United States’ FDA in 2020, under the brand name Orgovyx, for the treatment of advanced prostate cancer. Relugolix has also been studied in the symptomatic treatment of endometriosis. Relugolix is the first (and currently only) orally-administered GnRH receptor antagonist approved for the treatment of prostate cancer – similar therapies such as [degarelix] require subcutaneous administration – and therefore provides a less burdensome therapeutic option for patients who might otherwise require clinic visits for administration by healthcare professionals. In addition to its relative ease-of-use, relugolix was shown to be superior in the depression of testosterone levels when compared to [leuprolide], another androgen deprivation therapy used in the treatment of prostate cancer.
Relugolix is a Gonadotropin Releasing Hormone Receptor Antagonist. The mechanism of action of relugolix is as a Gonadotropin Releasing Hormone Receptor Antagonist, and Cytochrome P450 3A Inducer, and Cytochrome P450 2B6 Inducer, and Breast Cancer Resistance Protein Inhibitor, and P-Glycoprotein Inhibitor. The physiologic effect of relugolix is by means of Decreased GnRH Secretion.
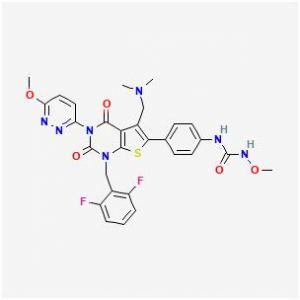
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
We have been commitment to supply the competitive price ,excellent products and solutions high-quality, at the same time as fast delivery for Reasonable price for Velpatasvir Manufacturer - Relugolix 737789-87-6 – CPF , The product will supply to all over the world, such as: Czech, Comoros, moldova, To meet the requirements of specific customers for each bit more perfect service and stable quality merchandise. We warmly welcome customers around the world to visit us, with our multi-faceted cooperation, and jointly develop new markets, create a brilliant future!
Product variety is complete, good quality and inexpensive, the delivery is fast and transport is security, very good, we are happy to cooperate with a reputable company!