Renewable Design for Lenalidomide Manufacturer - Captopril – CPF
Renewable Design for Lenalidomide Manufacturer - Captopril – CPF Detail:
Description
Captopril (SQ-14534) is a potent, competitive inhibitor of angiotensin-converting enzyme (ACE).
In Vitro
Captopril (SQ-14534) has been shown to have similar morbidity and mortality benefits to those of diuretics and beta-blockers in hypertensive patients. Captopril (SQ-14534) has been shown to delay the progression of diabetic nephropathy, and enalapril and lisinopril prevent the development of nephropathy in normoalbuminuric patients with diabetes[1]. An equimolar ratio of the cis and trans states of Captopril (SQ-14534) exists in solution and that the enzyme selects only the trans state of the inhibitor that presents architectural and stereoelectronic complementarity with its substrate binding groove[2].
MCE has not independently confirmed the accuracy of these methods. They are for reference only.
Clinical Trial
| NCT Number | Sponsor | Condition | Start Date |
Phase |
| NCT03179163 | Penn State University|National Heart, Lung, and Blood Institute (NHLBI) | Hypertension,Essential | July 20, 2016 |
Phase 1|Phase 2 |
| NCT03660293 | Tanta University | Diabetes Mellitus, Type 1 | April 1, 2017 |
Not Applicable |
| NCT03147092 | Centro Neurológico de Pesquisa e Reabiitação, Brazil | Hypertension|Blood Pressure | February 1, 2018 |
Early Phase 1 |
| NCT00252317 | Rigshospitalet, Denmark | Aortic Stenosis | November 2005 |
Phase 4 |
| NCT02217852 | West China Hospital | Hypertension | August 2014 |
Phase 4 |
| NCT01626469 | Brigham and Women´s Hospital | Type 2 Diabetes Mellitus | May 2012 |
Phase 1|Phase 2 |
| NCT00391846 | AstraZeneca | Heart Failure|Ventricular Dysfunction, Left | October 2006 |
Phase 4 |
| NCT00240656 | Hebei Medical University | Hypertension, Pulmonary | October 2005 |
Phase 1 |
| NCT00086723 | Northwestern University|National Cancer Institute (NCI) | Unspecified Adult Solid Tumor, Protocol Specific | July 2003 |
Phase 1|Phase 2 |
| NCT00663949 | Shiraz University of Medical Sciences | Diabetic Nephropathy | February 2006 |
Phase 2|Phase 3 |
| NCT01437371 | University Hospital, Clermont-Ferrand|Servier|LivaNova | Heart Failure | August 2011 |
Phase 3 |
| NCT04288700 | Ain Shams University | Infantile Hemangioma | October 1, 2019 |
Phase 4 |
| NCT00223717 | Vanderbilt University|Vanderbilt University Medical Center | Hypertension | January 2001 |
Phase 1 |
| NCT02770378 | University of Ulm|Reliable Cancer Therapies|Anticancer Fund, Belgium | Glioblastoma | November 2016 |
Phase 1|Phase 2 |
| NCT01761916 | Instituto Materno Infantil Prof. Fernando Figueira | Preeclampsia | January 2013 |
Phase 4 |
| NCT01545479 | Instituto de Cardiologia do Rio Grande do Sul | Renal Disease | January 2010 |
Phase 4 |
| NCT00935805 | Hospital de Clinicas de Porto Alegre|Conselho Nacional de Desenvolvimento Científico e Tecnológico|Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul, Brazil | Diabetes Mellitus|Arterial Hypertension | July 2006 |
|
| NCT00742040 | The Hospital for Sick Children | Heart Disease | August 2008 |
Phase 2 |
| NCT03613506 | Wuhan University | Radiotherapy Side Effect|Taking Captopril | October 25, 2018 |
Phase 2 |
| NCT00004230 | Northwestern University|National Cancer Institute (NCI) | Cancer | October 1999 |
Phase 3 |
| NCT00660309 | Novartis | Type 2 Diabetes Mellitus | April 2008 |
Phase 4 |
| NCT00292162 | NHS Greater Glasgow and Clyde | Chronic Heart Failure|Atrial Fibrillation | January 2007 |
Not Applicable |
| NCT01271478 | Coordinación de Investigación en Salud, Mexico | Inflammation|End-stage Renal Disease | August 2009 |
Phase 4 |
| NCT04193137 | Chongqing Medical University | Primary Aldosteronism | November 30, 2019 |
|
| NCT00155064 | National Taiwan University Hospital | Hyperaldosteronism | July 2002 |
Phase 4 |
| NCT01292694 | Vanderbilt University|Vanderbilt University Medical Center | Hypertension|Pure Autonomic Failure|Multiple System Atrophy | March 2011 |
Phase 1 |
| NCT00917345 | National Taiwan University Hospital|Novartis | Primary Aldosteronism | January 2008 |
|
| NCT00077064 | Radiation Therapy Oncology Group|National Cancer Institute (NCI)|NRG Oncology | Lung Cancer|Pulmonary Complications|Radiation Fibrosis | June 2003 |
Phase 2 |
Storage
| Powder |
-20°C |
3 years |
|
4°C |
2 years | |
| In solvent |
-80°C |
6 months |
|
-20°C |
1 month |
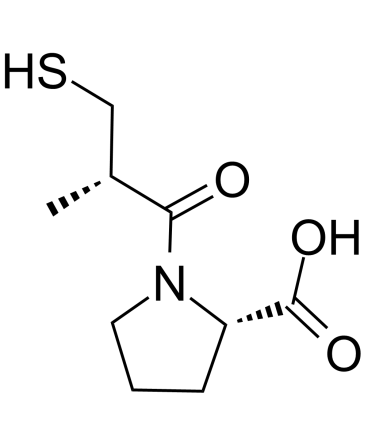
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
Our products are greatly acknowledged and reliable by users and may fulfill repeatedly shifting financial and social wants for Renewable Design for Lenalidomide Manufacturer - Captopril – CPF , The product will supply to all over the world, such as: Zambia, Buenos Aires, Austria, We strongly believe that technology and service is our base today and quality will create our reliable walls of future. Only we have better and better quality , could we achieve our customers and ourselves, too. Welcome customers all over the word to contact us for getting further business and reliable relationships. We are always here working for your demands whenever you need.
The factory technical staff not only have high level of technology, their English level is also very good, this is a great help to technology communication.











