Short Lead Time for Doxycycline Monohydrate For Syphilis - Sacubitril Hemicalcium – CPF
Short Lead Time for Doxycycline Monohydrate For Syphilis - Sacubitril Hemicalcium – CPF Detail:
| 沙库比曲半钙盐 | Sacubitril Hemicalcium | 1369773-39-6 | In-House |
| 沙库比曲钠盐 | 149690-05-1 | In-House | |
| LCZ-4 | 1426129-50-1 | In-House | |
| LCZ-7 | 1012341-48-8 | In-House | |
| LCZ-8 | 1012341-50-2 | In-House | |
| LCZ-9 | 149690-12-0 | In-House |
Background
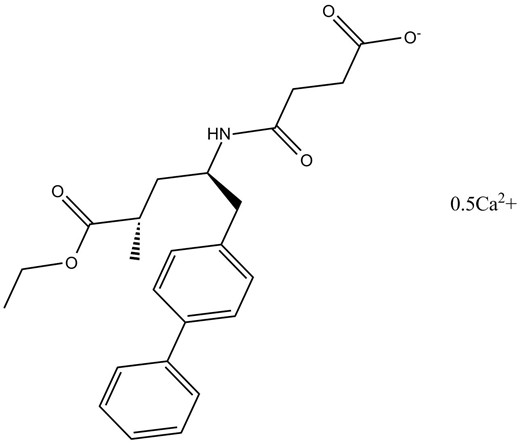
AHU-377 hemicalcium salt is a hemicalcium salt form of AHU-377. It is an inhibitor of neprilysin with IC50 value of 5 nM [1].
AHU-377 and the angiotensin II AT1 receptor antagonist valsartan compose LCZ696 in a 1:1 molar ratio. LCZ696 is an angiotensin receptor neprilysin inhibitor. It can reduce blood pressure and may be a novel drug for the treatment of heart failure. AHU-377 is a pro-drug, it can be converted by enzymatic cleavage of the ethyl ester into the active form LBQ657. It is reported that AHU-377(30 and 100 mg/kg, PO) can cause antihypertensive effect in a dose-dependent manner in DAHI-SS rats. But in the DOCA-salt hypertensive rats, it shows a weak reduction [2, 3].
References:
[1] Ksander GM, Ghai RD, deJesus R, Diefenbacher CG, Yuan A, Berry C, Sakane Y, Trapani A. Dicarboxylic acid dipeptide neutral endopeptidase inhibitors. J Med Chem. 1995 May 12; 38(10):1689-700.
[2] Voors AA, Dorhout B, van der Meer P. The potential role of valsartan + AHU377 ( LCZ696 ) in the treatment of heart failure. Expert Opin Investig Drugs. 2013 Aug;22(8):1041-7.
[3] Laxminarayan G Hegde, Cecile Yu, Cheruvu Madhavi et al. Comparative efficacy of AHU-377, a potent neprilysin inhibitor, in two rat models of volume-dependent hypertension. BMC Pharmacology 2011, 11(Suppl 1):P33.
Chemical structure
Proposal 18 Quality Consistency Evaluation projects which have approved 4, and 6 projects are under approving.

Advanced international quality management system has laid solid foundation for sales.

Quality supervision runs through the whole life cycle of the product to ensure the quality and therapeutic effect.

Professional Regulatory Affairs team supports the quality demands during the application and registration.
VIEW MORE
VIEW MORE
International cooperation

Domestic cooperation

Product detail pictures:

Related Product Guide:
We have been convinced that with joint attempts, the business enterprise between us will bring us mutual benefits. We could guarantee you product or service good quality and aggressive value for Short Lead Time for Doxycycline Monohydrate For Syphilis - Sacubitril Hemicalcium – CPF , The product will supply to all over the world, such as: Brazil, Tajikistan, Austria, We offer OEM services and replacement parts to meet the varying needs of our customers. We offer competitive price for quality products and we will make certain your shipment is handled quickly by our logistics department. We sincerely hope to have the opportunity to meet with you and see how we can help you further your own business.
This manufacturers not only respected our choice and requirements, but also gave us a lot of good suggestions, ultimately, we successfully completed the procurement tasks.











